Generally, it’s been fairly difficult to make anti-viral drugs. Often, a lot of the problem comes from the way viruses work. When they are outside the cell, they don’t have any active metabolic processes that can be targeted by drugs. And when they are inside the host cells, the metabolic processes they’re mostly using are ours. So it’s very hard to target the viruses without attacking the host. Compared to bacteria, viruses present a very small target.
Could a paper published in PLOS One called “Broad Spectrum Anti-viral Therapeutics” represent a penicillin moment for viruses ? A trailblazer that can transform our relationship with viruses ? To work this out, let’s take a good long look at what these anti-viral therapeutics are, and what they do. To do that, we have to talk about DRACO.
Are we talking about the DRACO from Harry Potter, or the dragon voiced by Sean Connery in Dragonheart ?
Whilst I compliment you on your knowledge of pop culture, you are wrong on both counts. DRACO is a handy acronym that stands for Double-stranded Ribonucleic acid Activated Caspase Oligomizer. DRACO is the drug that is being purported to extinguish all of those nasty viruses. To understand how it works, we need to take a look at how some of our most pernicious viruses work.
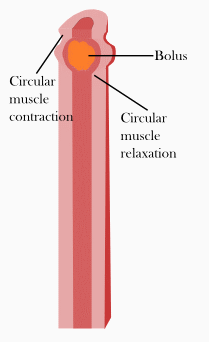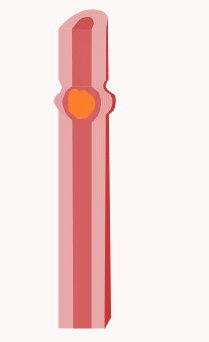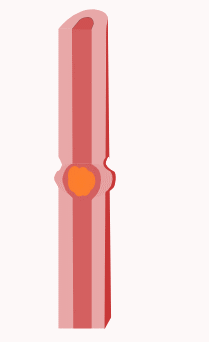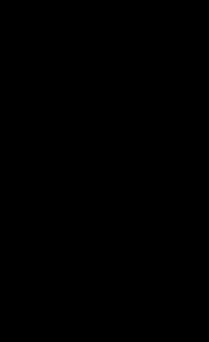
You may have heard about DNA, and how it’s genetic code is a blueprint for life. Your genome is encoded in your DNA. The nucleus of your cell holds all of the DNA, and acts like a library for your genetic code. When your cell needs a specific genetic code to make a certain protein, the nucleus makes an RNA copy of the appropriate gene and sends it out into the rest of the cell, where it can be used to construct a protein.
The goal of a virus is to enter a cell, and to hijack this process to make more viruses. Usually at some point, the virus will attempt to substitute it’s own genetic code for that of the host cell, tricking the host cell to make more viruses [1].
However, some viruses can store their genetic code using RNA only. Whilst RNA is less stable than the DNA, it means that these viruses can go straight to the cell machinery that translate RNA into protein, and get ahead with making virus based proteins.