Let us say that you are afflicted by the common cold, a set of symptoms that are triggered by a number of different rapidly evolving viruses for which there is currently no cure. The only way one could cure it is if you had a drug that could hit every single one of these viruses. In fact, if you were to describe the whole family of different viruses in terms of a rainbow, the kind of treatment you need is one that will hit the whole spectrum.
What you need is a broad spectrum antiviral.
I've previously talked about one class of antivirals known as DRACO's, which have a side effect of killing the virus by killing the cells they inhabit, effecting a scorched earth policy against the virus.
Today, we'll be looking at another drug that could potentially fight off a broad spectrum of viruses.
So how do we go about finding new drugs to fight viruses ?
I have previously talked (at some length) about how DRACO's were developed, based on examining natural processes, and developing a drug that manipulated those processes for our own ends. But this is not actually how many drugs are discovered. If we look at the history of antibiotic discovery, we find that many of them were discovered through testing every chemical available until we find one that does what we want it to do. As I like to call it "Throw S**t against the wall and seeing what sticks" approach to drug discovery. But that makes it sound too easy. You need to be very careful about the kind of shit you're using, and you need to be smart about the wall you're throwing it against. Otherwise you could spend you entire life throwing shit against a wall with naught to show for it except sticky hands and a vastly reduced social circle.
These researchers started out searching for chemicals that can prevent an engineered Vesicular Stomatitis Virus (VSV) entering into kidney epithelial cells extracted from the African Green Monkey, known as "Vero cells". These engineered viruses have a gene encoding luminescence, which means that when they successfully infect a Vero cell, they start emitting light. If the Vero cells don't emit light, and are still alive at the end of the experiment, then something has stopped the virus from infecting the cell. It is a relatively rapid system for quickly testing out drugs to stop viruses infecting cells.
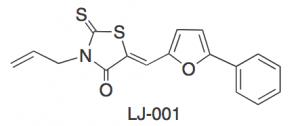
The researchers initially wanted to develop a new drug against Nipah virus, which is related to the VSV test virus used in this experiment. They created the "LJ-series" of compounds. The researchers don't go into the rationale behind their choice of compounds, but to be fair the only one they need is that these compounds haven't been tested before.
After what I presume was strenuous testing*, they eventually discovered a compound that stopped VSV from infecting Vero cells. This compound was named LJ-001.
So then the researchers tested it out against the Nipah virus, and found that this compound was just as effective at preventing Nipah virus from infecting Vero cells.
The key graph is below, shown in A, with the black and dotted lines representing the VSV and Nipah viruses respectively, and the dashed line showing the control cells that were not infected with viruses.
Panel B shows what happens over time if you add the VSV to Vero cells over a time course with and without LJ-001 and measure the numbers of viruses produced by infected cells. The LJ-001 puts a real crimp in the production of viruses.
In even better news, Panel C shows that LJ-001 will only start showing signs of toxicity at concentrations of log10 -4, which is the highest value shown in Graph A.
So this is good news. But it gets better. The researchers then tested to see whether this compound could prevent other viruses infecting Vero cells. It was effective against enveloped viruses like HIV, Influenza, Hepatitis C, and even Ebola. But when it came to Adenoviruses (which often cause the common cold) it didn't work. It was a shame, but it told the researchers something very important about LJ-001, and how it works.
Viruses like Influenza, HIV, Hepatitis C and Ebola have a coating of fatty lipids. Lipids coat the surface of human cells as well. The lipid envelope of theses viruses can fuse with the lipid surface of human cells to help the viruses enter the cells.
But adenoviruses don't have a lipid envelope, and enter human cells using a different system.
So the evidence suggests that LJ-001 works by preventing viruses enveloped with lipids from fusing with their targets on human cells.
So is LJ-001 binding to a target on the host cell, or on the virus ?
Panel A-C. The researchers added the LJ-001 before infection, and measured how well they infected the cells. The earlier they added LJ-001 to the virus, the less able it was to infect cells. If they added it just to the viruses before they infected the cells, it prevented the viruses from working. This strongly suggests that the LJ-001 acts directly on the viruses.
Panel D. They treated viruses such as the rift valley fever and Ebola with LJ-001 before using them to infect mice. Now, in real life viruses don't tend to take baths in antiviral drugs before infections, so don't assume that this graph will in any way represent how treatments would actually progress. It does however fully support the idea that LJ-001 is acting on the virus directly, and not the host.
Now that this has been established, the question arises as to what LJ-001 actually does to the membrane of the virus. The great thing about LJ-001 is that it naturally fluoresces at 510nm when it is dissolved in lipids, so they can actually measure how much LJ-001 insinuates within a membrane by seeing how fluorescent the membrane becomes.
Panel A. We can see an example of this. They have a set of Liposomes, which are basically pure lipid membranes with no virus or anything in them. When these are added to the LJ-001, we can see that there is a strong fluorescent signal with a wavelength spectrum centered on 510nm.
Panel B. They add differing concentrations of the liposomes to LJ-001. The luminescence signals from these indicate that the LJ-001 has dissolved within them, and thus become fluorescent.
Panel C They took Vero cells and added differing concentrations of LJ-001 to them, and then measured their fluorescent signal. Not only does this demonstrate that LJ-001 can enter the membranes of host cells, but that we can determine the amount of LJ-001 in the membranes of Vero cells using the fluorescence signal.
Panel D. Now we have the bioluminescent VSV again. Big luminescence signals indicate that it can successfully infect vero cells, low ones indicate no infection. We have two treatment groups, we have LJ-001, and the control. But in this experiment they added liposomes. These liposomes act like a sponge for the LJ-001, and every molecule they suck up is one hat can't attack a virus. So when they are added at higher concentrations, we can see that they prevent LJ-001 from working properly.
Panel E. However, if they did the same experiment where they added the LJ-001 to the viruses before they interacted with the vero cells, then no luminescent signal is recovered, indicating that no infections take place.
The researchers could now say with some confidence that LJ-001 affects the viral membrane. The question remained as to how it affected the membrane. What was this molecule doing ?
To get an idea about this , they took a closer look at the structure of LJ-001.
We can assume that different parts of this molecule are somehow attacking the enveloped of the virus. How do we find out which parts of the molecule are important, and which ones are not ?
The scientists performed a structure-activity study to work this out. They created a number of chemicals that were similar to LJ-001, except with a difference in the basic structure.
I'll give a quick example. You see on the far left of the structure, there is this big hexagonal ring of carbons. This part is very fatty, and thus can help the compound mix with oily lipids. But if we attach an acid to it, this will give the ring a charge, and make it less able to mix with oily lipids, and better able to mix with water molecules. So if we make a change like this, we can change the properties of different parts of a compound, and then see whether it is still able to fight off viruses. They created 26 of these compounds, and then tested them for their ability to attack viruses.
They were able to then work out which parts of this molecule allow it to attack viruses, but not human cells.
They found that:
- The portion of the molecule highlighted in green seemed to have no real purpose, they could add what they liked to it, and it didn't change the antiviral properties of the molecule.
- The blue portion of the molecule was essential to it's antiviral activity, and could tolerate no changes. The double bod that links it to the orange portion of the molecule is particularly important.
- As you can tell from the previous, the orange portion of the molecule plays an important role in relation to the blue part. Changes to it reduce the drugs effectiveness.
- Changes to the red portion of the molecule reduce the drugs effectiveness only if those changes give it a charge. This part of the molecule is essential for allowing the drug to enter the membranes of viruses.
What happens to the virus after it encounters LJ-001?
So the researchers now know which portions of LJ-001 are messing with the virus. But we still don't actually know about its target. all we know is that LJ-001 does "something" within the lipid envelope of the virus, and not much else.
The researchers sought to improve this situation by taking a much closer look at what this drug does to the rift valley fever virus.
They added the LJ-001 to a sample of virus, and then broke the virus up into it's constituent proteins and ran them on a western blot. In simple terms a western blot is a way for researchers to weigh different proteins found in the virus. If the LJ-001 is binding specifically to one of these proteins, they would become heavier, and this would show up on the western blot. However, none of the proteins they tested became any heavier, suggesting that LJ-001 had a different target. They repeated this experiment with a number of different viruses, and came up with the same results.
What stage of infection is LJ-001 stopping ?
The researchers took some nipah virus treated with LJ-001 and used it to infect some host cells. They added a fluorescent antibody designed to bind to Nipah virus. Cells with Nipah virus attached to them would end up glowing as a result of this antibody.
What they found was interesting. LJ-001 didn't actually prevent the virus from attaching to the surface of their target cells, even though it stopped the virus from actually infecting the cell.
To further delve into this, the researchers needed to take a deeper look at how viruses fuse with the cell membrane, in order to understand how LJ-001 interferes with this process.
The researchers used a specially engineered virus to study this process. This virus, descended from Nipah virus, was engineered to possess special enzyme fused into one of the key proteins within it. If the virus successfully fuses with it's host cell, this enzyme is released into the host cytoplasm. This enzyme reacts with chemicals within the host cell to turn it blue. So the researchers could tell whether the viruses successfully fused with the host cytoplasm by checking out which cells had turned blue.
Panel A shows that LJ-001 prevents virus cell fusion. But the question arises, if it can mess with viruses, could it do the same to host cells that had already been infected with virus ?
Viruses are known to be able to transfer themselves to new cells by causing the host cell to fuse with it's neighbour, creating cells that are known as "syncytia". They infected cells with this special strain of nipah virus, and then added them to uninfected cells in the presence of LJ-001. Panel B shows that they did not prevent the fusion of host cells.
This highlighted that LJ-001 only interferes with the virus lipid layer, and does not seem to damage the host. So the researchers asked themselves "What were the main differences between the lipid layer around the virus, and the lipid layer around a host cell ?"
One of the main differences between viruses and most living things is the presence of an active metabolism. To people particularly interested in classifying things, Viruses in the free living portion of their life cycle are not considered to be alive. If their membranes get damaged, they cannot repair them. Living cells however have an array of different ways to repair their cell membranes when they get damaged.
The researchers hypothesised that the LJ-001 was causing a small degree of damage to the living host cells, but these cells were able to repair it, as opposed to the viruses, which ended up "dead". If this was the case, then if they stopped the living repair processes of the living host cells, then they would render them vulnerable to the effects of LJ-001 in the way that viruses are naturally vulnerable.
Scroll up to Panel C and you will see how they tested this. They added TOFA, which prevents new lipids from being made, and thus prevents the lipid membrane of the host from being replenished with new lipids after damage has occurred. So they used a Toxilight assay, in which measures when cells leak adenylate kinase into the medium, which usually occurs when cells are dying.
They added LJ-001 to cells that had been treated with TOFA, and found that this treatment had made the host cells more likely to die off after LJ-001 treatment. This suggests that LJ-001 acts through damaging Lipid layers on all cells, and takes advantage of the lack of membrane repair systems within viruses.
Summary
- The discovery of LJ-001, a new antiviral is a great finding in itself. Even if it only affected Nipah virus, or VSV, it would be a cool discovery.
- The fact that it can hit the whole spectrum of enveloped viruses is brilliant.
- We know that it can attack some target within the membrane, and that the only way we currenlty know that viruses can overcome this resistance would be to either completely lose the envelope, or to develop membrane repair mechanisms like living cells.
Statistics
They use students T-test for all of their comparisons (according to their methods), which actually is not problematic for most of their analyses. Figure 2 A is the only ones where this is may be a problem, because they appear to compare more than two groups. This requires a correction for multiple comparisons, to avoid a type 1 error. The reason I don't think this will be much of a problem is because I decided to do my own Bonferroni correction (assuming they used n=10), which basically sets the p-value at a new level to take the multiple comparisons into account. This means that they would now need to get a p-value lower than 0.005 in order for them to be able to declare the results significant. For those experiments, they got p< 0.001.
So the paper's statistics are actually pretty good (for a microbiology paper) for the experiments in which they were used.
Lingering questions
- What is the LJ-001 doing to the virus membrane ? We know it is causing damage, but how is it doing it ?
- Will this actually be a viable treatment ? We have seen how the LJ-001 could be effective if the viruses soak themselves in it before infection ?
- Does the presence of any lipid layer make this treatment unfeasible ? We have seen how liposomes can nerf the effectiveness of this drug simply by being present. What will happen to this drug when it is put inside a body in which there are tons of lipid bilayers that will suck up the drug before it manages to even see a virus.
I will be analysing the follow up to this paper tomorrow.
Microbiology Twitter journal club will start this Tuesday, 8pm BST
follow the #microtwjc hastag.
*I do not know this for certain, but suspect the author list is as long as it is because so many of them must have worked on the screening process, and through no fault of their own turned up with nothing, but the lead authors want to acknowledge the sacrifice of the scientists who came before. This is the ultimate risk of "sh*t against the wall" research for academia.






















Do you think the alpha-beta unsaturated bond makes a covalent bond with some essential molecule?
ReplyDeleteIt has been suggested that this could happen. I address it more in my follow up. I think the western blot in this paper rules out the LJ-001 bonding to proteins within the membrane, so that rules them out. That leaves lipids and sugars, and if we go by the assumption that this molecule is primarily non-polar, then that will rule out sugars.
DeleteIt could bond with cholesterol. There is nothing in the paper that disputes that, but since cholesterol appears to be irrelevant to infection, it wouldn't be this effect that inactivates the virus.
Is that figure 4? that seems to measure "intactness" of the virion, not potential covalent modification of a viral protein...unless i'm not looking at it carefully. i can think of one example where the thiol of cysteine residue of a protein reacts (slowly reversibly) with the alpha-beta unsaturated bond of a drug. is it possible reducing conditions of the SDS-PAGE reversed the potential mod? (sorry, i'm not a chemist...) perhaps the hydrophobic moiety targets it to the viral membrane and it reacts with something crucial
DeleteIn figure 4 A, they show a western blot with Rift valley fever virus in which they show the two glycoproteins that form the coat of the virus, and nucleoprotein N, and the lack of shift in these individual proteins suggest that they aren't being bonded covalently by LJ-001.
DeleteActually, you raise a really good point about the reducing conditions. I hadn't thought of that. In this case, we can't rule out LJ-001 acting through covalent bonding to proteins, because they would be undone by DTT or whatever other reducing agent they were using. You're right !
I'm going to edit the follow up to this, thanks for your insight.
If you get the time, you should tune in to tomorrow's MicroTwJC discussion on Twitter. It's at 8pm BST. If you have a twitter account, you can join in.